Abstract
Introduction: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired blood disease characterized by hemolytic anemia, bone marrow failure, and thrombosis. Prior to the FDA approval of the C3 inhibitor pegcetacoplan, PNH treatment only included the C5 inhibitors (C5i) ravulizumab (RAV) and eculizumab (ECU) to decrease intravascular hemolysis and improve anemia. A Phase III trial of RAV in a treatment-naïve PNH population reported hemoglobin (Hb) stabilization (avoidance a >2 g/dL decrease in baseline Hb) occurred in 68% of RAV and 64% of ECU patients after 6 months. (Lee et al, 2019). However, real world (RW) evidence of the efficacy of these agents on Hb measures in clinical practice are lacking.
The objectives of this study were to describe the demographic, healthcare resource utilization (HCRU), and clinical characteristics of PNH patients prior to C5i therapy, and to examine changes in Hb levels from baseline to 6 months after treatment initiation in a PNH patient population using a RW electronic medical record (EMR) network.
Methods: This was a retrospective study of PNH patients aged ≥ 12 years seen between 2010-2021 within the TriNetX Dataworks USA Network, a federated EMR network of 68+ million de-identified patients. Included patients had a diagnosis of PNH on or before the index event of C5i therapy initiation. Patients were excluded if there was no visit recorded ≥6 months before the index event or if they had a diagnosis of atypical hemolytic uremic syndrome, myasthenia gravis, or neuromyelitis optica spectrum disorder. Demographic, HCRU, and clinical characteristics were captured for the year prior to therapy initiation. Hb results closest to the first treatment date and to the 6-month follow-up dates were used for Hb values. Mean and median Hb and distribution of Hb were calculated, as well as Hb stabilization (avoidance of a >2 g/dL decrease in baseline Hb) and response (Hb≥1 g/dL improvement) rates.
Results: Fifty-eight PNH patients treated with RAV and 124 patients treated with ECU met inclusion criteria. Among the RAV and ECU cohorts respectively, patients were on average 50 and 43 years old and 59% and 56% female. Both cohorts experienced an average of ~3 inpatient visits in the year prior to C5i initiation. Aplastic anemia (36% vs 43%) and myelodysplastic syndromes (3% vs 6%) were less common among the RAV vs ECU cohort. Previous ECU treatment occurred in 34 of 58 (59%) RAV patients. All ECU patients were treatment-naïve at baseline.
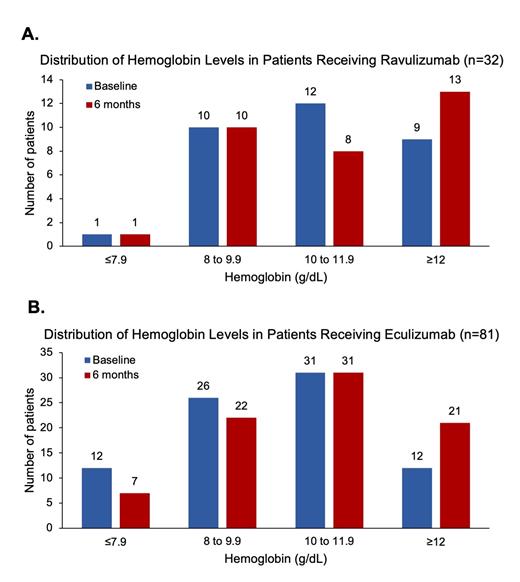
Hb results were available at baseline and 6 mo. in 50 and 32 RAV patients and in 110 and 81 ECU patients. The mean (SD) baseline and 6 mo. Hb values were 10.6 (2.0) and 10.7 (2.2) g/dL for the RAV cohort and 9.8 (2.2) and 10.4 (2.2) g/dL for the ECU cohort.
Analysis of baseline and 6 mo. Hb values showed trends toward Hb increase for both cohorts driven by the larger proportion of patients achieving Hb≥12 g/dL (Figure). While the proportion of patients with Hb<10 g/dL decreased in the treatment-naïve ECU cohort (47 to 36% at 6 mo.), it remained unchanged from baseline to 6 mo. (34%) in the RAV cohort. Hb stabilization and response at 6 mo. were observed in 94% and 34% of RAV and 93% and 51% of ECU patients. However, these do not take transfusion requirements into account, which may lower these results.
Conclusions: This analysis shows that patients treated with ECU had modest improvements in Hb measures after 6 mo., likely reflecting the effects of C5i in a treatment-naïve population. However, RAV treatment did not markedly alter Hb measures, perhaps due to this being mainly a cohort switching between two therapies with identical mechanisms of action. These RW Hb results suggest that despite C5i treatment, anemia was still largely persistent in this PNH cohort, possibly due to incomplete control of ongoing hemolysis, including extravascular hemolysis, which is not treated by these therapies.
Our current analysis has limitations that we plan to address: Subgroup analysis by prior treatment is planned to further characterize RAV efficacy. Transfusion requirements have not yet been incorporated into this analysis; however, their inclusion is likely to attenuate the observed Hb efficacy. Other labs, such as absolute reticulocyte count and LDH, will be incorporated to gain a more holistic picture of therapeutic response. Finally, HCRU encounters reflecting adverse events, such as breakthrough hemolysis, need to be analyzed to understand the real-world safety profile of these therapies.
Yeh: Apellis Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Kuranz: TriNetX: Current Employment; Apellis Pharmaceuticals, Inc.: Other: Payments from Apellis Pharmaceuticals to my institution TriNetX. Brzozowski: Apellis Pharmaceuticals, Inc.: Other: Payments from Apellis Pharmaceuticals to my institution TriNetX; TriNetX: Current Employment. Hoffman: Apellis Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Fishman: Apellis Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Chen: TriNetX: Current Employment; Apellis Pharmaceuticals, Inc.: Other: Payments from Apellis Pharmaceuticals to my institution TriNetX.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal